|
Atomic Bonds: Attraction of Ions. . Ionic bonds are atomic bonds created by the attraction of two differently charged ions. The bond is typically between a metal and a. non-metal. The structure of the bond is rigid, strong and often crystalline and solid. Ionic bonds also melt at high temperatures. Dis Show
Top 1: Ionic Bond ExamplesAuthor: examples.yourdictionary.com - 79 Rating
Description: Atomic Bonds: Attraction of Ions Ionic bonds are atomic bonds created by the attraction of two differently charged ions. The bond is typically between a metal and a. non-metal. The structure of the bond is rigid, strong and often crystalline and solid. Ionic bonds also melt at high temperatures. Dis
Matching search results: Fe(HCO3)2 - Iron(II) Hydrogen Carbonate; Fe(OH)2 - Iron(II) Hydroxide; Fe(MnO4) ...Fe(HCO3)2 - Iron(II) Hydrogen Carbonate; Fe(OH)2 - Iron(II) Hydroxide; Fe(MnO4) ... ...
 Top 2: What are Ionic Compounds? - Definition, Structure, Properties ...Author: byjus.com - 122 Rating
Description: What is Ionic Compound?. Ionic Compound Structure. Ionic Compound Examples. Ionic. Compound Properties. Ionic Character Formula. Frequently Asked Questions – FAQs. Which is an ionic compound?. What are the common ionic compounds?. What is the ionic bond example?. Is MgO an ionic compound?. What are the 2 parts of an ionic compound?. 1. Physical properties of ionic compounds. 2. Melting and boiling points of ionic compounds. 3. The solubility of ionic compounds. 4. Conduction of Electricity.
Matching search results: Ionic Compound Examples ... For example, the reaction between magnesium and chlorine. The magnesium atom has two electrons in its outermost shell.Mercury Element: Charge Of ElectronAtomic Model: Plaster Of Paris FormulaRutherford Model: What Is CorrosionIonic Compound Examples ... For example, the reaction between magnesium and chlorine. The magnesium atom has two electrons in its outermost shell.Mercury Element: Charge Of ElectronAtomic Model: Plaster Of Paris FormulaRutherford Model: What Is Corrosion ...
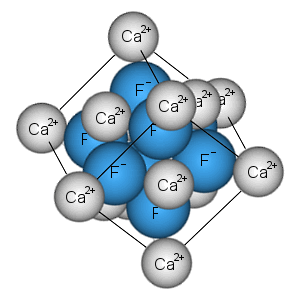 Top 3: Definition of Ionic Compounds - Chemistry Dictionary - ChemicoolAuthor: chemicool.com - 121 Rating
Description: What are Ionic Compounds?. Bonding in Ionic Compounds. The Formulas and Structures of Ionic Compounds. Electric Conductivity. Solutions of Ionic Compounds What are Ionic Compounds?Ionic compounds are compounds consisting of ions. Two-element compounds are usually ionic when one element is a metal.
Matching search results: Ionic compounds are compounds consisting of ions. Two-element compounds are usually ionic when one element is a metal and the other is a non-metal. Examples ...Ionic compounds are compounds consisting of ions. Two-element compounds are usually ionic when one element is a metal and the other is a non-metal. Examples ... ...
Top 4: 3.5: Ionic Compounds- Formulas and Names - Chemistry LibreTextsAuthor: chem.libretexts.org - 247 Rating
Description: Compounds Containing Only Monatomic Ions. Compounds Containing Polyatomic Ions. Compounds Containing a Metal Ion with a Variable Charge. Contributors and Attributions . Last updated Save as PDF Page ID37955. 6.9: Binary Ionic Compounds and Their Properties6.18: Ionic Compounds Containing Polyatomi
Matching search results: 25 July 2022 · The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic ...25 July 2022 · The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic ... ...
 Top 5: Ionic compounds | Definition, Properties, & Examples - TutorsAuthor: tutors.com - 133 Rating
Description: What is an ionic compound?. What are ionic compounds made of?. Ionic vs.. molecular compounds. Properties of ionic compounds. Ionic compound examples. Ionic compound formula What is an ionic compound?Ionic compounds are pure substances consisting of chemically bonded ions. Examples include two-eleme
Matching search results: Ionic compounds are pure substances consisting of chemically bonded ions. Examples include two-element compounds like table salt (NaCl) N a C l and ...Ionic compounds are pure substances consisting of chemically bonded ions. Examples include two-element compounds like table salt (NaCl) N a C l and ... ...
 Top 6: Examples of Ionic Compounds in Everyday Life - Science NotesAuthor: sciencenotes.org - 130 Rating
Description: Covalent and Ionic Compound Worksheet Examples of ionic compounds in everyday life include table salt, baking soda, lye, Epsom salt, and bleach.There are. many examples of ionic compounds in everyday life. Ionic compounds consist of atoms joined together by ionic bonds. Many ionic compounds are bina
Matching search results: 10 Mar 2021 · Examples of ionic compounds in everyday life include table salt, baking soda, lye, Epsom salt, and bleach. There are many examples of ionic ...10 Mar 2021 · Examples of ionic compounds in everyday life include table salt, baking soda, lye, Epsom salt, and bleach. There are many examples of ionic ... ...
 Top 7: ionic bond | Definition, Properties, Examples, & Facts | BritannicaAuthor: britannica.com - 112 Rating
Description: Entertainment & Pop CultureGeography &. Travel. Health & MedicineLifestyles & Social IssuesLiteraturePhilosophy & ReligionPolitics, Law & GovernmentScienceSports & RecreationTechnologyVisual. ArtsWorld HistoryOn This Day in HistoryQuizzesPodcastsDictionaryBiographiesSummari
Matching search results: ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ...
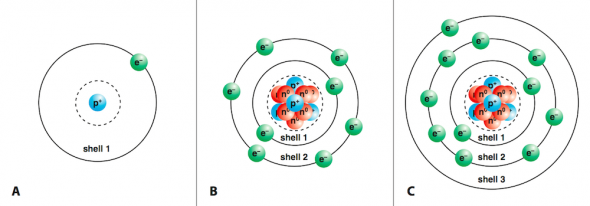 Top 8: Ionic Compounds - University of Hawaii at ManoaAuthor: manoa.hawaii.edu - 142 Rating
Description: Ion Formation Patterns. Salts are Ionic Compounds Electron ShellsElectrons are in constant motion outside of an atom’s nucleus. The electron shell is the region that the electrons travel in (see Fig. 2.21). Electron shells are labeled with numbers 1 through 7. Each shell holds an increasing number
Matching search results: Other examples of ionic compounds that combine in a ratio of one cation to one anion are sodium chloride (NaCl) and potassium iodide (KI). In comparison, Group ...Other examples of ionic compounds that combine in a ratio of one cation to one anion are sodium chloride (NaCl) and potassium iodide (KI). In comparison, Group ... ...
|

Related Posts
LATEST NEWS
Populer
About

Copyright © 2024 ShotOnMac Inc.




























